MU Health Care's Ellis Fischel Cancer Center plays an integral role in improving the lives of people battling cancer today and in the future.

Our doctors, scientists, health professionals and participants are leading the way to advancing treatments, discovering breakthroughs and working toward a cure through a number of types of clinical trials. Clinical trials are research studies that involve people who volunteer to take part in tests of new drugs, current approved drugs for a new purpose, or medical devices.
Ellis Fischel Cancer Center Clinical Trials Office, CTO, has overseen hundreds of trials and enrolled thousands of cancer patients. Past and present trials have included treatment, prevention, screening and supportive/palliative care studies. The CTO oversees several studies that are industry, institutionally and federally sponsored. These diverse trials and partnerships often give patients access to the latest cancer treatments and therapies before they become widely available.
About CTO and Our Mission
Our goal at CTO is to provide high-quality support to everyone involved in the clinical trial process. Our support involves the principal investigators (lead investigator), clinicians and, most importantly, the patients enrolled in trials. The purpose of CTO is to provide oversight, patient support and quality control for ongoing clinical trials at Ellis Fischel in order to advance MU Health Care’s mission to save and improve lives.
CTO team members assist with clinical trials from start to finish. Services provided include trial budgeting, protocol development, regulatory documentation management, trial research data collection and expert nurse clinicians.
Our Patients and their Clinical Trials Experience
Thousands of Ellis Fischel patients have volunteered to take part in clinical trials. Those patients play a vital role in the fight against cancer because without them, we would not have the treatment options we have today.
Patients enrolled in a clinical trial are assigned to one of the skilled and experienced CTO nurse clinicians who specialize in cancer care. Our nurse clinicians provide individualized support to each participant from beginning to end of the trial and often beyond.
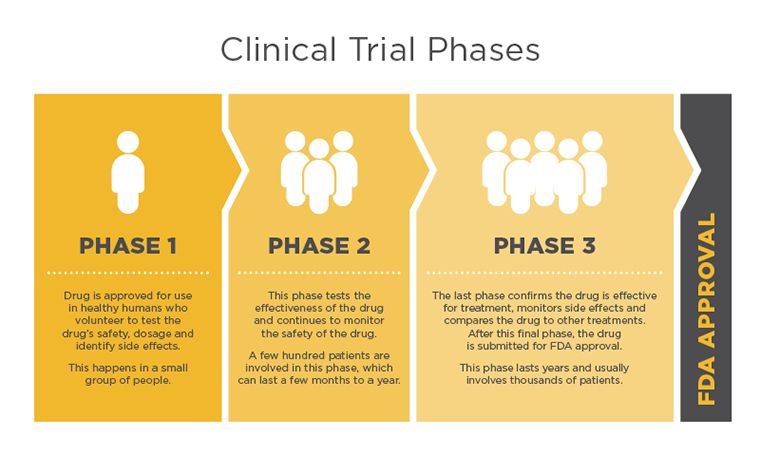
Clinical Trial Phases
The final step in the lengthy and thorough research process of any new treatment is conducting clinical trials in people. Clinical trials determine if the new treatment is safe, effective and works better than current standard treatments available.
All new treatments must go through clinical trial phases to be considered for Food and Drug Administration (FDA) approval. Each trial phase is designed to answers specific questions.
Phase I studies are usually the first that involve people. This phase assists in finding the highest dose of the new treatment that can be given safely, without serious side effects.
Phase II studies test to find out if the new treatment is effective. The goal in this phase is based on the response the clinicians are looking for. There could be several different outcome types: the cancer shrinks, disappears, stops growing, longer remission time or improved quality of life.
Phase III clinical trials compare the safety and effectiveness of the new treatment against the current standard treatment. Doctors do not yet know if the new treatment is better than the standard, so study participants are often picked at random to get either the standard treatment or the new one. Many phase III studies will not disclose to the doctor or the patient which treatment is being given. This type of study is called a double-blind study.
Phase IV studies look at drugs that have been approved by the FDA. Even with FDA approval and availability to patients, the drugs will continue to be monitored so the full effects of the treatment are known.
Clinical Trial Eligibility
Each trial has specific guidelines, known as eligibility criteria, which can vary from study to study. Eligibility criteria are used to determine if a patient would be a good fit for the trial. Enrolling patients with similar characteristics helps researchers achieve precise and meaningful data that will determine if the study was successful in achieving the desired outcome.
If there is a study of interest that you or a loved one may qualify for, or you have additional questions about a specific trial, contact one of the CTO nurse clinicians.
Clinical Trials Informed Consent
In order to take part in a clinical trial, patients must give their written informed consent after learning of the risks, benefits and other important information relevant to the study. The informing process includes discussion about any additional tests that may be required, costs of participation, inconveniences, as well as the rights and responsibilities of the participant. Throughout the study, patients will be informed as new information becomes available and may be asked to sign another informed consent if new benefits, risks or side effects are discovered.
Before signing the consent, the doctor and CTO clinical study coordinator will go over the study in detail with the prospective participant. At the end of the discussion, patients should understand the study’s purpose, procedures involved, treatment timeline, patient rights and possible risks and benefits.
Patients are usually sent home with a copy of the consent before signing so they can discuss it with their support team and gather any questions. Patients who decide to participate and meet the qualifications in the clinical trial will return to the hospital to meet with their physician or study staff to sign the consent form.
If the patient agrees to join the study, it is important to keep in mind that signing the informed consent form does not mean patients must stay in the study. Patients can leave the study at any time for any reason.
Additional Resources for Patients and Caregivers
Joining a clinical trial is an important decision. Below are resources that have additional information that might be helpful when deciding if a clinical trial is right for you and your family.